SARS-CoV-2 / COVID-19 Diagnosis and Confirmation Solutions









On This Page |
COVID-19 RDT Ag Testing | COVID-19 Molecular Testing | COVID-19 Molecular Testing | qPCR Instruments and Reagents | ddPCR Instruments and Reagents | COVID-19 Serology Testing |
For COVID-19 diagnosis and confirmation, Bio-Rad offers a 15-minute SARS-CoV-2 antigen test for rapidly diagnosing patients in laboratory and point-of-care settings. Our full range of molecular testing options for COVID-19 testing, includes both Real-Time PCR and Droplet Digital PCR (ddPCR). Real-time PCR is the gold standard in COVID-19 diagnostic testing, providing accessible, rapid results. The high sensitivity of ddPCR makes it well suited for population screening through pooled testing, and to aid in confirming negative results by other methods. For serological testing, we offer the Platelia SARS-CoV-2 Total Ab assay, which detects anti-nucleocapsid antibodies IgM, IgA, and IgG in one test to help identify individuals with an adaptive immune response to SARS-CoV-2.
Bio-Rad provides a full range of molecular testing options for COVID-19 testing, including both Real-Time PCR and Droplet Digital PCR (ddPCR). Real-time PCR is the gold standard in COVID-19 diagnostic testing, providing accessible, rapid results. The high sensitivity of ddPCR makes it well suited for population screening through pooled testing, and to aid in confirming negative results by other methods. For serological testing, we offer the Platelia SARS-CoV-2 Total Ab assay, which detects anti-nucleocapsid antibodies IgM, IgA, and IgG in one test to help identify individuals with an adaptive immune response to SARS-CoV-2.
Some products have limited regional availability. Please contact your local sales office contact your local sales office for any product availability questions.
COVID-19 RDT Ag Testing

Coronavirus Ag Rapid Test Cassette (Swab)
The Coronavirus Ag Rapid Test is a rapid test for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal swab or nasal swab specimens for the diagnosis of COVID-19 infection.
3 Quick Steps
In just three steps, the Coronavirus Ag Rapid Test detects the presence or absence of SARS-CoV-2 antigen in specimens collected at the first onset of symptoms.
Highly Specific and Sensitive Results
The Coronavirus Ag Rapid Test demonstrated high performance for specificity and sensitivity in a rigorous evaluation at seven test sites.
High Performance for Both Specimen Types
-
Nasopharyngeal Swab
-
99.6%
Specificity98.32%
Sensitivity
Nasal Swab
-
100%
Specificity97.25%
Sensitivity
Limit of Detection
1.15 x 102 TCID50/ml
-
Rapid Test: Results in 15 Min
-
1
Short sample preparation
-
2
Fast test procedure
-
3
Easy result interpretation
Antigen Detection

Nucleocapsid Protein (N)
antigen from SARS-CoV-2
Authorization
- CE-IVD
For more information, download the IFU.
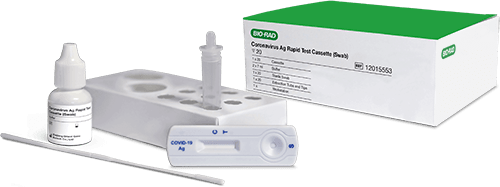
Coronavirus Ag Rapid Test Cassette (Swab)
Catalog #12015553
Learn more about our SARS-CoV-2 antigen rapid test and our world-class technical support.
Order Now Contact a Sales SpecialistCOVID-19 Molecular Testing
Reliance SARS-CoV-2 and Flu A/B RT-PCR Assay Kits
We offer two COVID-19 RT-PCR in vitro diagnostic tests that can detect SARS-CoV-2 RNA alone or simultaneously detect and differentiate SARS-CoV-2, influenza A, and influenza B RNA. We have performed a variant analysis of our SARS-CoV-2 kits, and their performance is unlikely to be affected by the variants. For details, see the IFU for each kit.
The US FDA has granted Emergency Use Authorization (EUA) for Bio-Rad's Reliance SARS-CoV-2 RT-PCR Kits in the U.S.*
- Highly sensitive one-step RT-PCR workflow; kit includes all reagents and controls (store at –20ºC)
- Specific panel designed with three sets of primers/probes in a single optimized multiplex assay
- Primer/probe sequences chosen for SARS-CoV-2 target the nucleocapsid (N) gene, which has higher viral specificity and may have lower mutation risk than the spike (S) protein gene
- Compatible with the most popular PCR instrument platforms (Bio-Rad CFX Systems and AB7500 Systems)
- Flexibility to address varying throughput, with 96-well and 384-well plate formats
Reliance SARS-CoV-2 RT-PCR Kit (IVD)
We now offer a CE-marked, COVID-19 RT-PCR in vitro diagnostic test that can detect SARS-CoV-2 RNA. We have performed a variant analysis of the SARS-CoV-2 kit, and its performance is unlikely to be affected by the currently circulating variants. For details, see the IFU for the kit.
The Reliance SARS-CoV-2 RT-PCR Kit (IVD) is a molecular in vitro diagnostic test targeting the virus nucleocapsid N1 and N2 genes to specifically detect the novel coronavirus.
- Highly sensitive one-step RT-PCR workflow; kit includes all reagents and controls (store at –20ºC)
- Specific panel designed with three sets of primers/probes in a single optimized multiplex assay
- Primer/probe sequences chosen for SARS-CoV-2 target the nucleocapsid (N) gene, which has higher viral specificity and may have lower mutation risk than the spike (S) protein gene
- Compatible with popular PCR instrument platforms (Bio-Rad CFX96 Dx System and AB7500 Fast Dx System)
- The assay is not affected by known coronavirus variants as determined by in-silico analysis
- Validated with 96-well plate format
This product is CE Marked with limited regional availability. Check with your local sales office.
SARS-CoV-2 Droplet Digital PCR (ddPCR) Kit
Kit Contents:
- 2019-nCoV CDC ddPCR Triplex Probe Assay
- 1-Step RT-ddPCR Advanced Kit for Probes
- SARS-CoV-2 Positive and Negative Standards
- SARS-CoV-2 Positive and Negative Standards
The Bio-Rad SARS-CoV-2 ddPCR Kit is a powerful diagnostic tool in the battle against COVID-19, with sensitivity and precision beyond that of qPCR assays.
The US FDA has granted Emergency Use Authorization (EUA) for Bio-Rad's SARS-CoV-2 ddPCR Kit in the U.S.*
The test is a partition-based endpoint RT-PCR test intended for the qualitative detection of nucleic acids from SARS-CoV-2 in nasopharyngeal anterior nasal and mid-turbinate nasal swab specimens, as well as nasopharyngeal wash/aspirate and nasal aspirate specimens from patients suspected of having COVID-19 by their healthcare provider.
The SARS-CoV-2 ddPCR Kit is optimized for use on Bio-Rad QX200 or QXDx AutoDG Droplet Digital PCR Systems. Analytic sensitivity is very high, calculated as 0.260 cp/µl to 0.351 cp/µl (cp = copies) for genetic markers, N1 and N2, using different extraction kits, without any significant loss in specificity.
- High sensitivity and precision in low viral abundance samples
- Resistant to inhibition often seen in RT-PCR testing
- Single-well test — 3 sequences aligned to CDC markers: SARS-CoV-2 N1 and N2 genes, human RPP30 gene
Molecular Standards and Controls for SARS-CoV-2
For Research Use Only
-

SARS-CoV-2 Standard
The SARS-CoV-2 Standard contains synthetic RNA transcripts of 5 SARS-CoV-2 gene targets and Human Genomic DNA.
-

SARS-CoV-2 Negative
The SARS-CoV-2 Negative contains genomic DNA at 75,000 copies/ml formulated in a synthetic matrix.

For CE-IVD Use
-

SARS-CoV-2 Positive Run Control
The SARS-CoV-2 Positive and Negative Run Controls are manufactured with synthetic RNA transcripts containing five gene targets and Human Genomic DNA.
-

SARS-CoV-2 Negative Run Control
The SARS-CoV-2 Negative Run Control contains Human Genomic DNA and is negative for SARS-CoV-2.
Molecular Standards and Controls for SARS-CoV-2
For Research Use Only
-

SARS-CoV-2 Standard
The SARS-CoV-2 Standard contains synthetic RNA transcripts of 5 SARS-CoV-2 gene targets and Human Genomic DNA.
-

SARS-CoV-2 Negative
The SARS-CoV-2 Negative contains genomic DNA at 75,000 copies/ml formulated in a synthetic matrix.

For CE-IVD Use
-

SARS-CoV-2 Positive Run Control
The SARS-CoV-2 Positive and Negative Run Controls are manufactured with synthetic RNA transcripts containing five gene targets and Human Genomic DNA.
-

SARS-CoV-2 Negative Run Control
The SARS-CoV-2 Negative Run Control contains Human Genomic DNA and is negative for SARS-CoV-2.

Please contact us for more infomarmation about how to order Molecular Standards and Controls for SARS-CoV-2.
Molecular Standards for SARS-CoV-2

For Research Use Only
-
SARS-CoV-2 Standard
The SARS-CoV-2 Standard contains synthetic RNA transcripts of 5 SARS-CoV-2 gene targets and Human Genomic DNA.
-
SARS-CoV-2 Negative
The SARS-CoV-2 Negative contains genomic DNA at 75,000 copies/ml formulated in a synthetic matrix.

-
Multiplex Molecular Run Controls
SARS-CoV-2, Flu, RSV Run Controls
The SARS-CoV-2, Flu, RSV Run Controls allow laboratories to evaluate day-to-day and lot-to-lot variation of their multiplexed molecular assay and to test for operator proficiency.
-

Have questions about our COVID-19 Test Assays?
Speak with an expert about your COVID-19 Test Assays questions.
Real-Time PCR Instruments and Reagents
CFX Real-Time PCR Detection Systems are utilized by many testing laboratories for the detection of SARS-CoV-2 (COVID-19). CFX Maestro Software provides superior data collection, statistical analysis, and data visualization capabilities.

Real-Time PCR Detection Systems for Research (RUO)
CFX Real-Time PCR (qPCR) Detection Systems
Quickly set up runs and monitor amplification traces in real time on the integrated touch screen, or use CFX Maestro Software to program runs and analyze data remotely.
- Solid-state optics with up to 5-channel multiplexing
- Accurate results with volumes as low as 3 µl
- Internal system qualification and system test software, automated data quality control, and validation tools
- Integrate with LIMS using built-in LIMS file management
- CFX Maestro Software, Security Edition for U.S. FDA 21 CFR Part 11 compliance

Real-Time PCR Detection Systems for In Vitro Diagnostics (IVD)
CFX96 Dx Systems
CFX96 Dx Systems are CE-IVD marked in compliance with the E.U. diagnostic medical device manufacturing standards for use in human in vitro diagnostics.*
- Up to 5-target multiplexing with Reliance One-Step Multiplex Supermix, reduces sample and reagent use
- Quickly and accurately analyze and validate data with CFX Manager Dx Software
- Reaction volumes: CFX Dx, 10–50 µl; CFX Dx Deep Well, 10–125 µl
- Microtube formats: 96-well plates, 8-well strips
Real-Time PCR Reagents & Consumables
Reliance One-Step RT-qPCR Supermix
Robust, reliable one-step RT-qPCR supermix, optimized for sensitive application of up to 5 targets directly from RNA.
Optimized for sensitive amplification of up to 5 targets directly from RNA.
- Ideal for viral and pathogen detection
- Single-tube, 4x ready-to-use supermix
- Multiplex up to 5 targets
- 24-hour room temperature stability for automated workflows

Selector Tools
Use our selectors to find the best reagents, plates, tubes, seals, and accessories for your PCR and qPCR experiments.


PX1 PCR Plate Sealer
Efficiently seals microplates for PCR amplification to deliver reliable data by minimizing sample evaporation during thermal cycling.
Droplet Digital PCR Instruments and Reagents
Droplet Digital PCR Systems
Bio-Rad’s Droplet Digital PCR Systems provide ultrasensitive and absolute nucleic acid quantification. Droplet Digital PCR is particularly useful for low-abundance targets, targets in complex backgrounds, or monitoring subtle changes in target levels that cannot be detected with real-time PCR.
-
QX200 AutoDG Droplet Digital PCR System for Research (RUO)
- Simplifies ddPCR workflow and ensures consistency
- Generates droplets for 96 ddPCR reactions in 45 minutes
- One AutoDG Instrument can supply 4–5 droplet readers
- HEPA-filtered enclosure reduces contamination risk
-
QXDx AutoDG ddPCR System for In-Vitro Diagnostics (IVD)
- First FDA-cleared ddPCR system for use with QXDx BCR-ABL %IS Kit
- Ultra-low quantification without standard curves
- Droplet partitioning reduces amplification bias
- Automated droplet generator, 96-reaction throughput


Droplet Digital PCR System Consumables
Purchase droplet generation oil, cartridges, gaskets, plates & seals, plus pipette tips, and more, designed for Droplet Digital PCR Systems.
-
Droplet Digital PCR System Supermix
One-Step RT-ddPCR Advanced Kit for Probes for use with the COVID-19 ddPCR Assay.

Droplet Digital PCR System Consumables
Purchase droplet generation oil, cartridges, gaskets, plates & seals, plus pipette tips, and more, designed for Droplet Digital PCR Systems.
-

Droplet Digital PCR System Supermix
One-Step RT-ddPCR Advanced Kit for Probes for use with the COVID-19 ddPCR Assay.
COVID-19 Serology Testing
Platelia SARS-CoV-2 Total Ab Assay
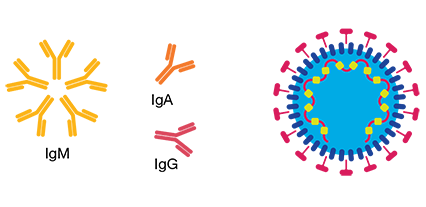
In clinical tests, the Bio-Rad Platelia SARS-CoV-2 Total Ab test demonstrated >99% specificity for SARS-CoV-2 while showing no cross-reactivity to non-SARS coronaviruses or other medical conditions. In a study of PCR-positive patients, the assay detected antibodies in 100% of patients that had samples collected and tested between 9 and 22 days after onset of symptoms.
This blood-based immunoassay, designed for in vitro diagnostic testing, is one of the first total antibody assays to have obtained U.S. Emergency Use Authorization to test for total antibodies (IgM, IgA, IgG) in a single assay. In addition, the test has met requirements for applying the CE-mark in Europe.
FDA Emergency Use Authorization
This test has been authorized by the FDA under an EUA for use by authorized laboratories. The test has not been FDA cleared or approved. The test has been authorized only for the presence of total antibodies against SARS-CoV-2, not for any other viruses or pathogens. The test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
For more information, download the IFU.
This test can be used as an aid in diagnosing SARS-CoV-2 infections when RT-PCR is not used as a first-line diagnostic tool, as when samples are collected more than 8 days after symptom onset. The test can also be used for suspected COVID-19 infections, particularly in patients hospitalized with severe symptoms who have tested negative with RT-PCR.
The Platelia SARS-CoV-2 Total Ab assay detects total anti-nucleocapsid antibodies (IgM, IgA and IgG) to SARS-CoV-2 with high sensitivity (92% sensitivity for patients tested ≤ 8 days after symptom onset and 100% for patients tested >8 days after symptom onset).
This blood-based immunoassay, designed for in vitro diagnostic testing, is the first serology assay with U.S. Emergency Use Authorization to test for total antibodies in a single assay. The test has also met requirements for applying the CE-Mark in Europe.
The Platelia SARS-CoV-2 Total Ab assay is not licensed in Canada, but is authorized pursuant to Section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19.
Learn more about using the Platelia SARS-CoV-2 Total Ab assay for Surveillance or contact a Bio-Rad sales specialist. contact a Bio-Rad sales specialist.
For more information, download the IFU.
For more information, download the IFU.
Platelia SARS-CoV-2 Total Ab Assay
Catalog #12013798 — Platelia SARS-CoV-2 Total Ab, 480 tests
Catalog #72710 — Platelia SARS-CoV-2 Total Ab, 96 tests
Learn more about our Platelia SARS-CoV-2 Total Ab assay, testing instruments and world-class technical support.
Order Now Contact a Sales Specialist Contact a Sales Specialist Contact a Sales Specialist
EVOLIS Automated Testing Platform
The Platelia SARS-CoV-2 Total Ab assay can be used with automated and manual immunoassay platforms for the detection of anti-SARS-CoV-2 IgM, IgA, and IgG. The assay is recommended for use on the validated Bio-Rad EVOLIS System and manual systems (IPS/PR4100/PW41), and it can also be run on other validated manual and automated platforms.
See EVOLIS Platform
EVOLIS Automated Testing Platforms
The Platelia SARS-CoV-2 Total Ab assay can be used with automated and manual immunoassay systems for the detection of anti-SARS-CoV-2 IgM, IgA and IgG. While the assay is recommended for use on the Bio-Rad validated, fully-automated EVOLIS Systems or stand-alone systems (IPS/PR4100/PW40), it can also be run on other validated manual or automated platforms.
See EVOLIS PlatformLegal Notice:
The Reliance SARS-CoV-2 RT-PCR Kit (IVD) is CE Marked for sale outside the U.S., with limited regional availability. Please check with your local sales office. This test is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal swab specimens from patients suspected of COVID-19. The CE-IVD-marked kit is compatible with the Bio-Rad CFX96 Dx System and Applied Biosystems 7500 Fast Dx Real-Time PCR System.
This Reliance SARS-CoV-2 RT-PCR Assay Kit has not been FDA cleared or approved. This test has been authorized by the FDA under an EUA for use by the authorized laboratory. This test has been authorized only for the detection of nucleic acid from SARS CoV-2, not for any other viruses or pathogens, and this test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
The CFX96 Dx System is intended for use in the fields of research and human in vitro diagnostics. The system is CE-IVD marked in compliance with the European Union diagnostic medical device manufacturing standards.
Europe: For in vitro diagnostic use. The CFX96 Dx System and CFX96 Deep Well Dx System meet the requirements of the In Vitro Diagnostic Medical Devices Directive (98/79/EC). The CE IVD, CFX96 Dx and CFX96 Deep Well Dx Systems are for distribution and use in specific European countries only (Austria, Belgium, Bulgaria, Croatia, Denmark, Estonia, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Malta, Netherlands, Poland, Portugal, Slovenia, Spain, and Sweden).
USA: For In Vitro Diagnostic Use. The CFX96 Dx and CFX96 Deep Well Dx Systems are registered with the US FDA as Class II 510(k) exempt devices.
China: The CFX96 Deep Well Dx System is registered for In Vitro Diagnostic use.
Worldwide: The CFX96 Dx and CFX96 Deep Well Dx Systems are registered for In Vitro Diagnostic use in the following countries: Argentina, Australia, Belarus, Bolivia, Brazil, Brunei, Chile, Colombia, Costa Rica, Dominican Republic, Egypt, Hong Kong, India, Laos, Morocco, New Zealand, Norway, Pakistan, Philippines, Qatar, S. Korea, Saudi Arabia, Singapore, Switzerland, Taiwan, Thailand, The Netherlands, Turkey, United Kingdom, Ukraine, Vietnam, and Yemen.
These products and/or their use are covered by claims of U.S. patents, and/or pending U.S. and non-U.S. patent applications owned by or under license to Bio-Rad Laboratories, Inc. Purchase of a product includes a limited, non-transferable right under such intellectual property for use of the product for internal research and diagnostic purposes only. No rights are granted for use of a product for commercial applications of any kind, including but not limited to manufacturing, quality control, or commercial services, such as contract services or fee for services. Information concerning a license for such uses can be obtained from Bio-Rad Laboratories. It is the responsibility of the purchaser/end user to acquire any additional intellectual property rights that may be required.
Kits and reagents are sold for research use only. Not for use in diagnostic procedures.
EVOLIS is a trademark of Bio-Rad Europe, GmbH in certain jurisdictions.
EVOLIS and EVOLIS TWIN PLUS are trademarks of Bio-Rad Europe, GmbH in certain jurisdictions.
BIO-RAD is a trademark of Bio-Rad Laboratories, Inc. in certain jurisdictions.
Scroll