Coronavirus Therapeutic Development & Manufacturing Solutions









Coronavirus
Therapeutic Development

On This Page |
Lead Discovery | Cell Line Development | Therapeutic Efficacy Testing | Process-Scale Therapeutic Purification | Therapeutic Quality Control | Post-Treatment Monitoring | More Solutions |
The identification and development of efficacious therapeutics to treat coronavirus infections is a major challenge demanding reduced time to market. Whether developing a novel therapeutic or re-evaluating on-market drugs, Bio-Rad has solutions for all phases of the process, from early development to preclinical and clinical studies through manufacturing and quality control.
Some products have limited regional availability. Please contact your local sales office for any product availability questions.
These pages list our product offerings for professional use only. Please contact your Bio-Rad local representative to check the availability in your country. contact a Bio-Rad sales specialist to check the availability.
Lead Discovery for Coronavirus Therapeutics
The development of novel biologic therapeutics to treat coronavirus infection starts with lead discovery, which includes the identification of native human or recombinant binding antibodies to viral protein targets.

Bio-Plex Immunoassay Development Kits & Reagents for Therapeutic Antibody Discovery
Discover, detect, and quantitate binding antibodies to viral proteins using Bio-Plex COOH Beads and Coupling Kits. Couple viral proteins to the beads and pull down antibodies for further development.
Learn MoreLead Discovery with the ZE5 Cell Analyzer

Characterize patient-derived neutralizing antibodies and study lead candidate effects on inflammation during therapeutic development with the ZE5 Cell Analyzer.
- High-parametric immunophenotyping
- Rapid discovery of neutralizing antibodies
- Evaluation of T cell-stimulating viral epitopes
- Detection of pro-inflammatory cytokines and cytokine receptors
Flow Cytometry Publications
Immunophenotyping of Peripheral Blood on the ZE5 Analyzer (PDF 384 KB)
Fast and Comprehensive Immunotyping of T-Cell Populations (PDF 392 KB)
Cross-neutralization of SARS-CoV-2 by a Human Monoclonal SARS-CoV Antibody. Pinto D et al. Nature 2020 May 18
Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Grifoni A et al. Cell 2020 May 14
Cell Line Development for Coronavirus Therapeutics
Cell line development is a key workflow in early process development to ensure maximal expression of the correct molecule and long-term stability of the cell line. Bio-Rad provides solutions for all steps of cell line development, confirmation, and maintenance.
Cell Line Creation: Transfection
Electroporation is a reliable, efficient transfection method adaptable to a wide variety of cell types. Consult our transfection protocol library for optimized protocols for every cell line.

Gene Pulser & MicroPulser Electroporation Systems
These electroporation instruments are suitable for all cell types, featuring programs for manual operation, preset protocols, user protocols, and an optimization protocol. Modifiable parameters include time constant, actual voltage applied, pulse interval, pulse time, and waveform selection.
Gene Pulser/Micropulser Electroporation Cuvettes are high-quality electroporation cuvettes that yield reproducible and consistent gene delivery to valuable transfection samples.
Our universal electroporation buffer is formulated for high transfection efficiency.
Browse the Transfection Protocol Library
Learn MoreCell Line Screening: Edit Confirmation & Genotyping
After transfection and primary selection, transformed cells are analyzed for correct genome edits by PCR. Additional assays may include determination of transgene copy number variation (CNV) and confirmation of cell line genomic stability.
Droplet Digital PCR Systems and Assays for Cell Line Genomic Analysis
Droplet Digital PCR is the ideal method for genome edit detection, CNV determination, and assessment of cell line genomic stability.

The QX200 AutoDG Droplet Digital PCR System provides sensitive detection of positive clones as well as characterizing the expected frequency of homologous recombination. It allows reproducible determination of gene vector copy number to assess the stability and safety of your cell lines to assure productivity.
Bio-Rad offers a comprehensive portfolio of Digital PCR Assays and Kits for numerous applications including:
-

ddPCR Assays Brochure (PDF 1.2 MB)
Learn More
Publication Direct Quantification of Genome Editing Efficiency from Whole Cells Using SingleShot Cell Lysis Buffer and ddPCR Genome Edit Detection Assays (PDF 211 KB)
Cell Line Screening: Clonal Selection, Isolation, and Stability
Flow cytometry and cell sorting can be used to analyze and isolate cells for a particular phenotype. In cell line development, initial screening of transformed cells on a cell analyzer is followed by pool enrichment or single-cell sorting to isolate a stable, high-yielding production cell line. Flow cytometry is also used for monitoring cell line phenotype stability throughout its lifetime, and for assessing thawed cells from frozen master cell banks.
Flow Cytometry and Antibodies for Cell Line Screening & Isolation
Cell Line Phenotyping with the ZE5 Cell Analyzer

The ZE5 Cell Analyzer is an innovative flow cytometer with flexible configurations to meet a broad range of experimental complexities and throughput needs – accessible for novice flow cytometry users yet flexible enough for flow cytometry professionals.
- Automation/robotics-friendly for high-throughput production
- Integrated 96/384/deep-well plate sample loader
- Multi-parameter analysis - up to five lasers and 30 detectors for 27 colors
Flow Cytometry Antibodies

Bio-Rad Antibodies.com provides a large catalog of highly cited antibodies and the ability to provide custom monoclonal generation in just 8 weeks.
Search for high-quality flow cytometry antibodies using key parameters like target species, host species, isotype, and clone of interest. Additional search criteria include antibody specificity and format.
Find Flow Cytometry AntibodiesEfficacy Testing for Coronavirus Therapeutic Development
A significant challenge in therapeutic development is characterizing cellular immune response and identifying the key mediators of efficacy. Studying immunogenicity and pharmacokinetics / pharmacodynamics (PK/PD) in treatment groups can include antibody characterization and cell phenotyping.
Protein Profiling: Multiplex Immunoassays
Protein profiling in viral disease can yield markers for treatment efficacy, and characterizing the immunogenicity of therapeutics is critical to proving their safety and efficacy. Cytokine profiling is particularly important in COVID-19 therapeutic development.
Bio-Plex Multiplex Immunoassays for Cytokine & Chemokine Profiling

To characterize host response to coronavirus disease and help monitor the effectiveness of potential therapeutics, cytokines and chemokines can be quantitated from serum or other samples using Bio-Plex Immunoassay Panels, as recently described in Lancet (Huang C et al. 2020), in a study of clinical features of patients infected with COVID-19.
Bio-Plex Multiplex Immunoassays use Luminex magnetic beads for the quantitation of over 450 biologically relevant targets in a 12.5 µl sample: markers for inflammation, immune response, and more. Choose assays in premade or custom configurations, or develop your own assays for new targets.
Learn MoreBioassay Development for Protein Therapeutics: Immunogenicity & PK/PD
Determining immunogenicity and PK/PD of protein therapeutics is a key step to assessing their safety and ADME. Bio-Rad has a large catalog of antibodies to many of the therapeutic antibodies known to modulate the immune system, providing the ability to assay anti-drug-antibody response.
Antibodies for Immune Response and Inflammation Monitoring
Browse antibodies to profile immune response to COVID-19 infection and monitor virus-induced inflammation. Choose from thousands of antibodies against targets including:
- Innate immune response to viral infection: pattern recognition receptors
and toll-like receptors - Targeted adaptive immune response against the virus: T cell and B cell markers
- Inflammatory response: cytokines and chemokines
Anti-Idiotypic Antibodies
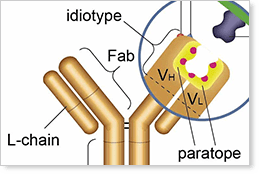
Bio-Rad is an expert manufacturer of highly specific, high affinity anti-idiotypic antibodies to support preclinical research, clinical trials, and patient monitoring.
These antibodies are made using the HuCAL® recombinant monoclonal antibody library and a novel phage display method to generate fully human antibodies in Fab and immunoglobulin forms.
Learn MoreRapid Custom Antibody Generation with HuCAL® Technology
Get highly specialized antibodies for coronavirus research and therapeutic development
- Fab antibodies against your target antigens in as little as 8 weeks
- Conversion to fully human IgG and IgM serological control antibodies
- Anti-idiotypic antibodies against your biologic drug for bioanalytical assays
Purification Solutions for Coronavirus Therapeutic Development & Manufacturing
For the development of a purification strategy for your therapeutic, we offer lab-scale chromatography systems and chromatography resins that can scale from lab to commercial-scale purification. Bio-Rad’s unique process chromatography resins provide purification benefits spanning a range of biomolecules.

NGC Chromatography Systems for Lab-Scale Therapeutic Purification
Browse our NGC Chromatography System configuration options for biomolecule purification to support your therapeutic development at the research, process-development, and laboratory-scale levels.
-

Fast resin screening of optimal purification conditions (PDF 227 KB)
-

Novel selectivities and workflows to streamline mAb purification processes (PDF 2.3 MB)

Bioprocess Resins for Commercial-Scale Therapeutic Purification
Bio-Rad’s unique process chromatography resins provide purification benefits spanning a range of biomolecules. Choose from new selectivity and high capacity chromatography techniques to maximize overall process efficiencies.
-

Efficient antibody purification with mixed-mode resins (PDF 2.4 MB)
-

Achieve higher yield and purity from sharper mAb elution profiles (PDF 395 KB)
-

Purification of large biomolecules with high-capacity ion exchanger Nuvia HP-Q resin (PDF 206 KB)
Can we answer a question for you?
For inquiries regarding purification solutions for coronavirus therapeutic development and manufacturing, please contact us.

Process Chromatography Resources
Purification resources to support you at each phase of process development in the downstream purification of biotherapeutics.

Process Chromatography Resources
Purification resources to support you at each phase of process development in the downstream purification of biotherapeutics.
Quality Control Solutions for Coronavirus Therapeutic Development & Manufacturing
Biologic therapeutics are complex medicines and must undergo rigorous testing to ensure consistency and safety. Our tools for biopharmaceutical quality control include quantitative systems for impurity testing.
Impurity Testing: Residual Host Cell DNA (HCD) and Host Cell Protein (HCP)
The FDA requires the quantitation and reporting of host cell DNA (HCD) and protein (HCP) in biologics due to their effects on efficacy and toxicity of the final product. HCD and HCP levels are also critically monitored during process development and validation.
Droplet Digital PCR Host Cell DNA (HCD) Assays

Droplet Digital PCR provides a highly sensitive method for direct quantification of residual HCD to ensure adherence to the FDA guideline of < 100 pg/dose. Our ddPCR Residual DNA Quantification Kit is optimized for HCD assays:
- Highly precise, femtogram-level quantification of residual CHO or E. coli DNA
- Direct quantification without DNA purification steps
- Inhibitor tolerance to in-process low-pH and high-salt sample matrices
- Available optimized supermix free of detectable E. coli, CHO, mouse, human, and yeast DNA
PublicationA Direct Droplet Digital PCR Method for Quantification of Residual DNA in Protein Drugs Produced in Yeast Cells
Hussain M et al. J Pharm Biomed Anal. 2016;123:128‐131
Biologics Analysis Workflow for Therapeutic Impurity Testing
The Biologics Analysis Workflow is a suite of products designed and validated to assess the purity or identity of biological products in a GMP regulatory environment and can be used to support HCP assays. The Biologics Analysis Workflow includes:

- GS-900 Calibrated Densitometer
- Image Lab Image Acquisition and Analysis Software*
- A electrophoresis starter kit with a Criterion Cell, TGX Gels, buffers, protein standards, and stains — enough for 10 runs
* Optional security module available for 21 CFR Part 11 compliance.
Vector Copy Number: Dosing Regimens for Cell Therapies
If your therapeutic is cell-based, then there may be a need for accurate vector copy number (CNV) determination. CNV is an important characterization when trying to achieve ideal dosing, as it has implications in therapeutic efficacy and risk. The high-precision absolute quantification of ddPCR enables the detection of small-fold copy number changes, providing confidence in discriminating between integration events beyond 3 copies.
Droplet Digital PCR Copy Number Assays

Our ddPCR Copy Number Determination Assays offer high precision and resolution to easily discriminate between small changes in copy number, e.g. a 1.2-fold change in CNV from 5 to 6 copies:
- Wet-lab validated assays available for more than 700 targets – FAM or HEX
- Guaranteed to work when used as instructed
- Validation data available for review and download
- Instant assay design for custom targets
-

ddPCR Assays for Mutation Detection and Copy Number Determination (PDF 1.2 MB)
-

Third-Generation PCR: A Novel Way to Accurately Measure Copy Number Variations in Human Genome (PDF 1.9 MB)
Post-Treatment Monitoring Solutions for Coronavirus Therapeutics
The precise quantification of viral particles is a critical indicator of patient response in clinical trials and post-approval studies. Droplet Digital PCR provides an ultra-sensitive and highly-precise absolute quantification of nucleic acid sequences.
Viral Load Monitoring: Measuring Viral Titer Reduction in Treatment Group
The 2019-nCoV CDC ddPCR Triplex Probe Assay is a quantitative assay for three sequences aligned to the three CDC-designated COVID-19 markers: the SARS-CoV-2 N1 and N2 genes and the human RPP30 gene.
SARS-CoV-2 Droplet Digital PCR (ddPCR) Kit
The Bio-Rad SARS-CoV-2 ddPCR Kit is a powerful diagnostic tool in the battle against COVID-19, with sensitivity and precision beyond that of qPCR assays.
The US FDA has granted Emergency Use Authorization (EUA) for Bio-Rad's SARS-CoV-2 ddPCR Kit in the US.*
The test is a partition-based endpoint RT-PCR test intended for the qualitative detection of nucleic acids from SARS-CoV-2 in nasopharyngeal anterior nasal and mid-turbinate nasal swab specimens, as well as nasopharyngeal wash/aspirate and nasal aspirate specimens from patients suspected of having COVID-19 by their healthcare provider.
The SARS-CoV-2 ddPCR Kit is optimized for use on Bio-Rad QX200 or QXDx AutoDG Droplet Digital PCR Systems. Analytic sensitivity is very high, calculated as 0.260 cp/µl to 0.351 cp/µl (cp = copies) for genetic markers, N1 and N2, using different extraction kits, without any significant loss in specificity.
- High sensitivity and precision in low viral abundance samples
- Resistant to inhibition often seen in RT-PCR testing
- Single-well test — 3 sequences aligned to CDC markers: SARS-CoV-2 N1 and N2 genes, human RPP30 gene
More Coronavirus Vaccine & Therapy Solutions
*Legal Notice:
The Bio-Rad SARS-CoV-2 ddPCR Kit is intended for use by qualified clinical laboratory personnel specifically trained and instructed in ddPCR techniques and in vitro diagnostic procedures, for use under the Food and Drug Administration’s Emergency Use Authorization in the U.S. and as an RUO kit outside the U.S.
These products and/or their use are covered by claims of U.S. patents, and/or pending U.S. and non-U.S. patent applications owned by or under license to Bio-Rad Laboratories, Inc. Purchase of a product includes a limited, non-transferable right under such intellectual property for use of the product for internal research and diagnostic purposes only. No rights are granted for use of a product for commercial applications of any kind, including but not limited to manufacturing, quality control, or commercial services, such as contract services or fee for services. Information concerning a license for such uses can be obtained from Bio-Rad Laboratories. It is the responsibility of the purchaser/end user to acquire any additional intellectual property rights that may be required.
Kits and reagents are sold for research use only. Not for use in diagnostic procedures.
Scroll